“Indian FDA” to be proposed in new bill
Other aims of the bill include creating a separate chapter of the clinical trials sector, which will introduce strict penal provisions for the payment of compensation, registration and the creation of ethics committees.
According to The Economic Times, health ministry officials say that the bill aims to introduce a comprehensive legislation for drugs and cosmetics, as well as strengthen the Indian drug manufacturing industry.
The proposed regulatory body
The proposed body would be headed by the health secretary and include secretaries of seven related ministries as members, as well as nominees and experts in the field.
The Central Drug Authority would have the power to review licenses granted to cosmetics and suspend or cancel those which did not meet requirement.
A previous attempt was made to introduce the idea in Parliament six years ago, but was defeated.
Cutting through the tangle
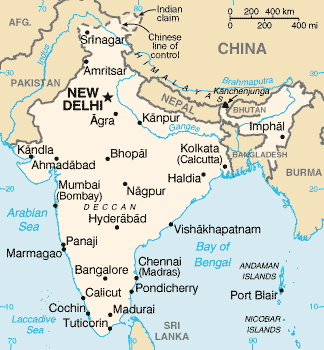
The Indian parliament is interested in creating a centralized licensing body in part because of a need to centralize the manufacture, sale and distribution of drugs.
Currently, the system has a cumbersome method by which a new drug is approved for marketing by the Central Drugs Standard Control Organization (CDSCO) but a license for its distribution needs to be issued by individual states.
The tangle is made worse by the fact that many states do not have officials with the necessary skills to evaluate a drug or cosmetic product before issuing a license.
Mashelkar Committee
The new bill is based in part on the recommendations of the Mashelkar Committee, a group assigned by the CDSCO to address problems with India’s existing drug regulations and enforcement system.
Regarding the group, the CDSCO states on their website: “There has not been a comprehensive review of the Drugs & Cosmetics Act 1940 since its enactment, although Rules have been amended from time to time to keep them up to date.”
“There is also a national concern regarding the problem of spurious drugs. It is important to see all the issues in an integrated manner.”



![Latest developments from the South Korean beauty market. [Getty Images]](/var/wrbm_gb_food_pharma/storage/images/_aliases/wrbm_tiny/publications/cosmetics/cosmeticsdesign-asia.com/headlines/brand-innovation/korea-focus-able-c-c-kolmar-and-more-in-this-k-beauty-round-up/17357973-1-eng-GB/Korea-focus-Able-C-C-Kolmar-and-more-in-this-K-beauty-round-up.jpg)

![Able C&C has furthered its partnership with Japanese discount chain Daiso with new makeup launch. [A'pieu]](/var/wrbm_gb_food_pharma/storage/images/_aliases/wrbm_tiny/publications/cosmetics/cosmeticsdesign-asia.com/headlines/brand-innovation/a-pieu-and-daiso-launch-exclusive-2-makeup-line/17339117-1-eng-GB/A-pieu-and-Daiso-launch-exclusive-2-makeup-line.jpg)
![Down Under Enterprises is setting sights on the Asian market as environmental sustainability and traceability become increasingly important. [Getty Images]](/var/wrbm_gb_food_pharma/storage/images/_aliases/wrbm_tiny/publications/cosmetics/cosmeticsdesign-asia.com/headlines/market-trends/down-under-enterprises-shifts-focus-to-china-as-environmental-sustainability-traceability-come-into-the-spotlight/17304932-1-eng-GB/Down-Under-Enterprises-shifts-focus-to-China-as-environmental-sustainability-traceability-come-into-the-spotlight.jpg)
![News updates from Shiseido, Dr.Ci:Labo, Sephora, and more. [Shiseido]](/var/wrbm_gb_food_pharma/storage/images/_aliases/wrbm_tiny/publications/cosmetics/cosmeticsdesign-asia.com/headlines/brand-innovation/updates-from-shiseido-dr.ci-labo-sephora-and-more/17334944-1-eng-GB/Updates-from-Shiseido-Dr.Ci-Labo-Sephora-and-more.jpg)

![Clariant has underscored the importance of localisation strategies and distribution capabilities in China with beauty trends evolving at a rapid pace. [Getty Images]](/var/wrbm_gb_food_pharma/storage/images/_aliases/wrbm_tiny/publications/cosmetics/cosmeticsdesign-asia.com/article/2024/04/16/clariant-emphasises-importance-of-localisation-in-the-era-of-viral-trends/17327969-1-eng-GB/Clariant-emphasises-importance-of-localisation-in-the-era-of-viral-trends.jpg)
