The findings were recently announced at the 2025 Colour Materials Research Presentation in Japan.
The pigments, named CaCoGe2O6 and CaCoSi2O6, are said to have the same stability and safety as conventional inorganic pigments while achieving superior vibrancy and vividness.
According to FANCL, makeup products generally use synthetic, or tar-based, dyes — artificially produced colourants that deliver colours, vividness, stability and uniformity, which are difficult to do so with natural pigments. However, synthetic pigments are well-documented to cause allergic reactions.
Inorganic pigments are colourants derived from non-living natural resources, such as soil, minerals and metal compounds, that are gentle on the skin. While stable and safe, they have the drawback of lacking colour vibrancy and tending to result in a somewhat dull finish.
“We believe that for cosmetics that come into direct contact with the skin, not only colours and textures are important, but also gentleness to the skin. Inorganic pigments excel at expressing subdued colours and are safe ingredients that can be used with peace of mind.
“However, there have been challenges in expressing more vivid colours. To solve this problem, we have been working to develop inorganic pigments that are both safe and vivid.
“With ‘gentleness to the skin’ as our number one priority, we are engaged in product development and research every day, with the desire to deliver products that will satisfy all those who enjoy makeup,” said Nao Kataoka, Makeup Development Group, Cosmetics Research Laboratory, General Research Institute at FANCL Corporation.
Going forward, the company will utilise the technology and experience gained through this research to develop makeup products using inorganic pigments to “brighten the daily lives of as many consumers as possible”.
The pyroxene structure
To develop a new material that is both safe and vivid, the researchers worked on replacing elements in complex oxides that can be coloured in a variety of shades while leveraging the advantages of inorganic pigments, particularly focusing on the pyroxene structure.
It is one of the main structures of pyroxenes — silicate minerals abundant in many igneous rocks. Known to be resistant to heat and light, the pyroxene structure has stable properties and can exhibit a variety of colours depending on the elements it contains.
Therefore, the researchers used CaMgGe2O6, a complex oxide with a pyroxene structure composed of elements that are harmless to the human body and the environment, as a base, and tested metal elements that would provide the colour for a new red inorganic pigment.
By replacing magnesium in CaMgGe2O6 with cobalt and firing it at a high temperature of 1200 degrees, they managed to synthesise CaCoGe2O6, an inorganic pigment with a very vivid reddish-purple colour.
As germanium contained in CaCoGe2O6 has few examples of use in cosmetics, the researchers considered replacing it with silicon, a semi-metallic element that is commonly used. However, they found that it would be difficult to synthesise it using the same method.
Through the investigation of a different synthesis method, they succeeded in creating CaCoSi2O6, a pink inorganic pigment.
Surpassing existing red pigments
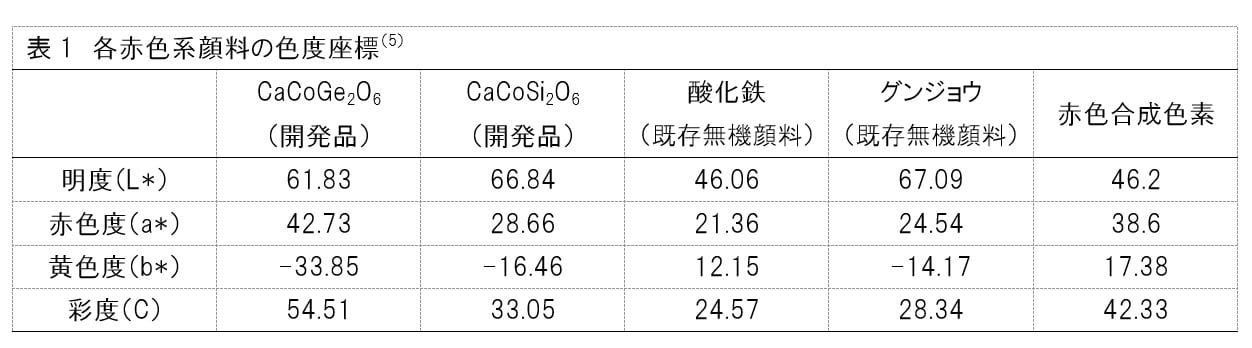
The researchers compared iron oxide and ultramarine, which are red inorganic pigments that are currently widely used in cosmetics, against the newly developed CaCoGe2O6 and CaCoSi2O6.
The L*a*b* colour system, a device-independent model representing colours visible to the human eye, was used for evaluation.
Specifically, the system comprises three indexes — L* is a measurement for the brightness of a colour with a value of 0 to 100 (the bigger the value, the brighter the colour); a* shows the redness of a colour (the larger the positive value, the stronger the redness, and the larger the negative value, the stronger the greenness); and b* tells the yellowness of a colour (the larger the positive value, the stronger the yellowness, and the larger the negative value, the stronger the blueness).
Additionally, saturation is calculated from the a* and b* values using the formula [√(a*)2+(b*)2].
Based on the results, the researchers confirmed that CaCoGe2O6 and CaCoSi2O6 possess vividness and redness values surpassing that of iron oxide and ultramarine.