Promotional Features
Uniquely stable emulsions revealed through freeze-fracture transmission electron microscopy
By: Christine Mendrok-Edinger, Karina Hecker and Melanie Waeckel, DSM Nutritional Products Ltd., Kaiseraugst, Switzerland; Stephan Dähnhardt-Pfeiffer, Microscopy Services, Dähnhardt GmbH, German
Freeze-fracture transmission electron microscopy (TEM) is a special technique that provides deep insight into emulsion structures. Researchers first used simple emulsions and TEM to compare two different emulsifiers often used in sunscreens: PEG-100 stearate and AMPHISOLK (or a special grade of ) potassium cetyl phosphate (PCP).
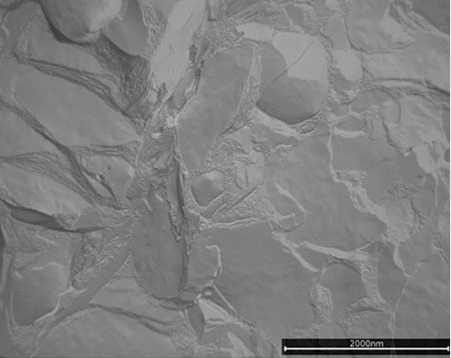
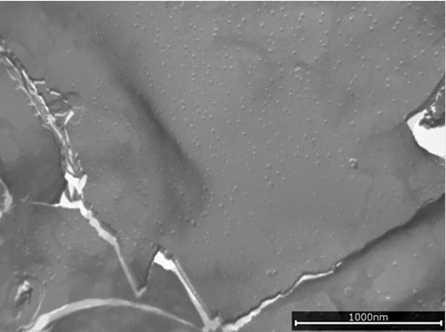
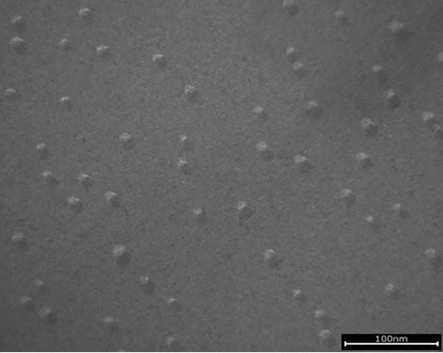
Both emulsifiers formed liquid crystalline lamellar (LCL) structures and created a gel network throughout the aqueous phase (see Figure 1 and Figure 2), but still the two emulsion structures look different. PEG-100 stearate led to many smaller, smooth areas of LCL structures with uneven edges, while the specific grade of PCP gave rise to rather large, smooth LCL areas with smooth edges and sharp corners. Additionally, the small and round micro-domains visible on top of the lamellar structure seemed specific to the special grade of PCP (see Figure 3 and Figure 4).
More complex sunscreens were then evaluated using freeze-fracture TEM. Three formulations either with glyceryl stearate citrate as emulsifier or PEG-100 stearate or the specific grade of PCP were tested.
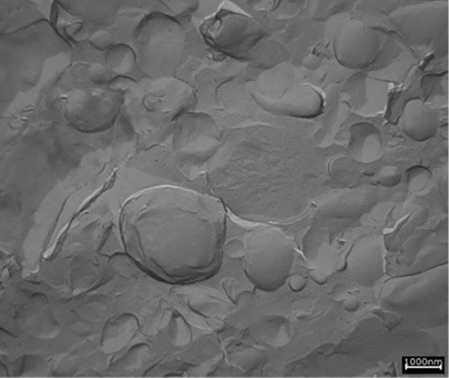
All three emulsifiers formed LCL structures (see Figure 5, Figure 6 and Figure 7) and revealed their gel network throughout the aqueous phase. In the cases of PEG-100 stearate and glyceryl stearate citrate, the structures found were mainly lamellar, whereas the specific grade of PCP also showed a significant number of droplets/vesicles. These vesicles consisted of emollient surrounded by PCP and co-emulsifier, and also show liquid crystalline structures within the vesicles; these areas were referred to as liquid crystalline vesicular (LCV) structures.
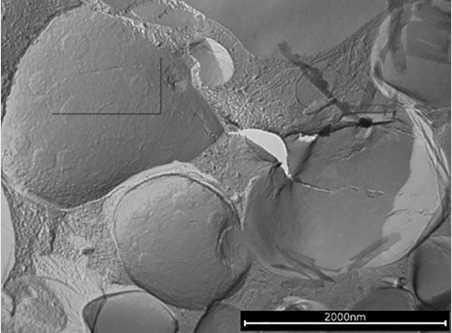
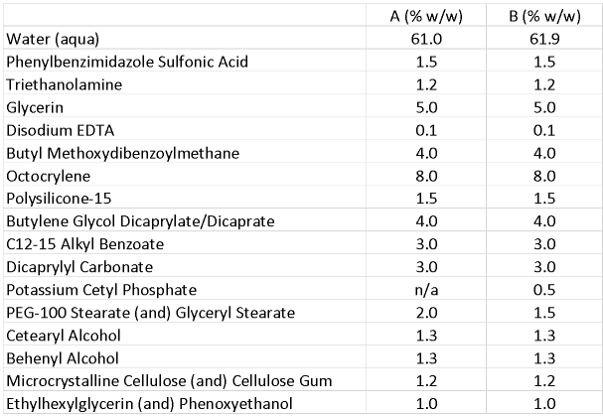
Next, PEG-100 stearate as a primary emulsifier was combined with the specific grade of PCP as a secondary emulsifier at a use level of 0.5% in an SPF 30 sunscreen emulsion (see Formula 1). Even when the specific grade of PCP was used as a secondary emulsifier the emulsion showed an increased number of LCVs. In this complex formulation, round, structured micro-domains were again found on top of the lamellar layers and especially on top of the vesicular structures (see Figure 8 and Figure 9).
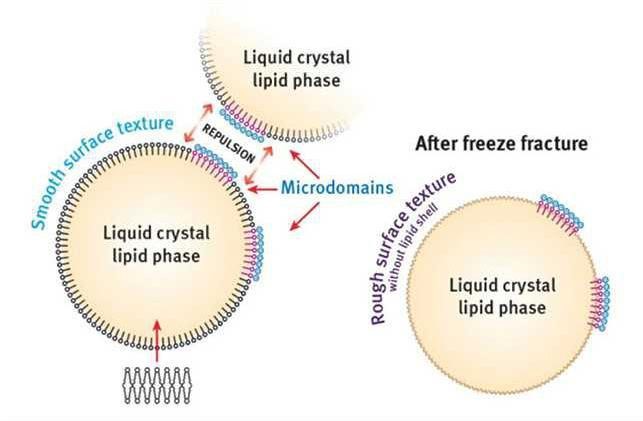
The micro-domains, visible as round-shaped spots with a smooth surface, are most likely self-organized PCP molecules on the surface of lipid vesicles that remained on the surface after freeze-fracture. The polar head groups of PCP give the network a high negative charge in these areas. This charge distribution leads to a repulsion of vesicles that would otherwise converge (see Figure 10); such repulsion of vesicles stabilizes the emulsion.
Stabilization power of emulsifiers
Sunscreens are complex systems and it is challenging to stabilize their high oil content along with UV filters,
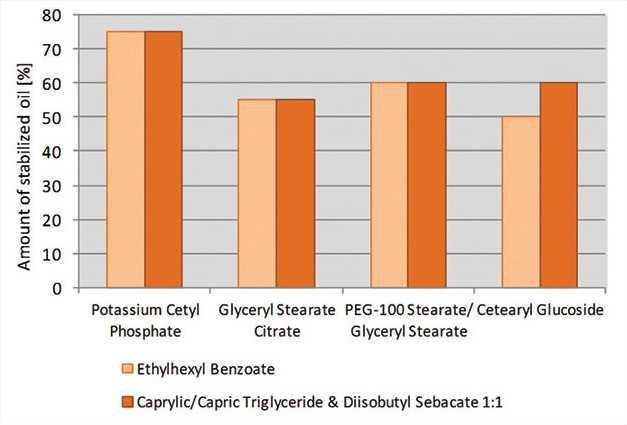
especially during elevated temperature testing. The specific grade of PCP revealed a high potential to stabilize polar and medium polar emollients; at a concentration of 1%, it stabilized up to 75% oil (see Figure 11). Other emulsifiers tested stabilized between 50% and 60% oil.
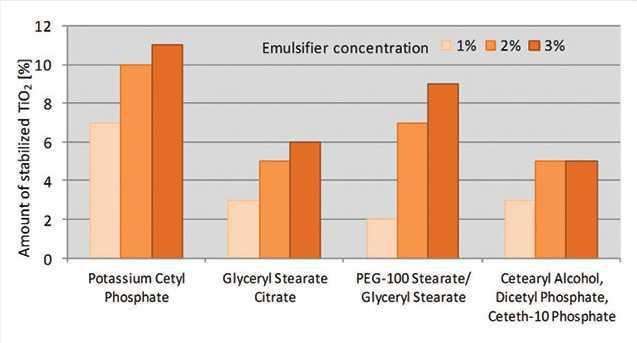
The specific grade of PCP also exhibited high potential to stabilize titanium dioxide; at a 1% concentration, it stabilized up to 7% titanium dioxide, while other emulsifiers stabilized formulations of 2% to 3% titanium dioxide. Further, at a 3% concentration, the specific grade of PCP could stabilize up to 11% titanium dioxide, while the other emulsifiers tested stabilized 5% to 9% titanium dioxide (see Figure 12).
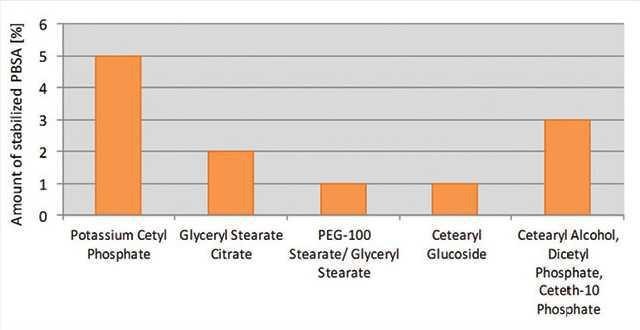
Additionally, the specific grade of PCP revealed a high potential to stabilize phenyl benzimidazole sulfonic acid (PBSA), a water-soluble UVB filter: a 1% concentration of the specific grade of PCP stabilized 5% PBSA, whereas the other emulsifiers could only stabilize between 1% and 3% (see Figure 13).
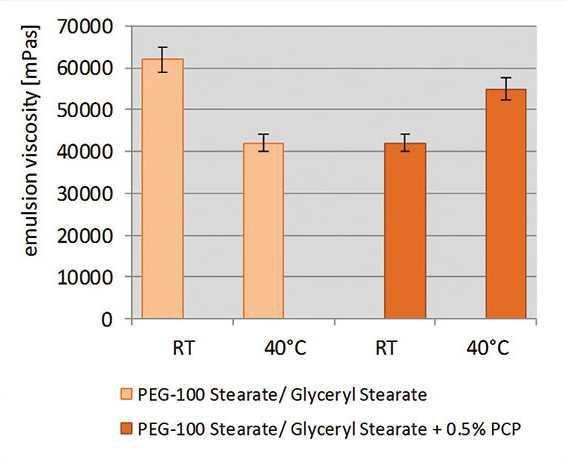
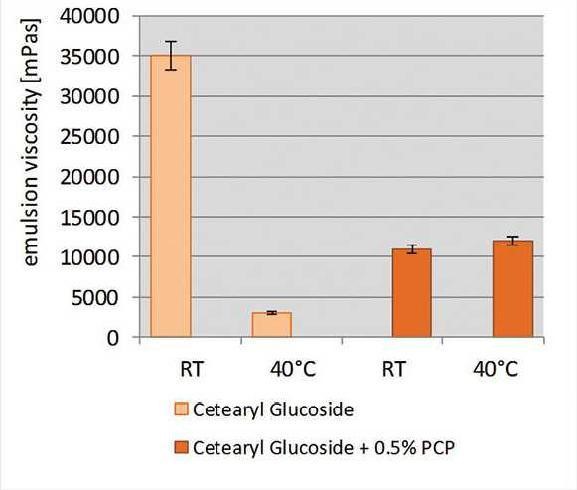
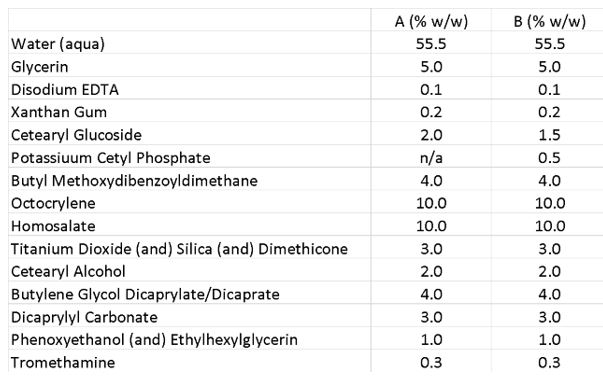
The ability of the specific grade of PCP to co-stabilize formulations against thermal stress also was investigated using PCP as the secondary emulsifier. Sunscreen emulsions with high oil loads and a targeted SPF 30 were formulated based on PEG-100 stearate (see Formula 1) or cetearyl glucoside (see Formula 2).
With a single emulsifier, these formulas were not stable when stored at 40°C. The specific grade of PCP at 0.5% was then incorporated; to maintain the overall amount of emulsifier in formulas, the primary emulsifier level was reduced when adding the specific grade of PCP. To analyze the stability of the formulations, the viscosity was measured at certain time points during six months of storage at 40°C. Any drop in viscosity indicated changes in emulsion structure—a clear indication of less stable emulsion systems (see Figure 14 and Figure 15).
The PEG-100 stearate formulation began to separate after four months of storage. The cetearyl glucoside formulation separated after five weeks of storage. However, formulations containing 0.5% of the specific grade of PCP were stable for one year at 40°C. The specific grade of PCP therefore significantly improved the thermal resistance of the formulas, even at low concentrations of PCP.
In conclusion, the presented research reveals the specific grade of PCP to be a unique emulsifier and the authors illustrated how even small amounts of this unique grade of PCP can optimally stabilize emulsions that have high loads of emollients, UV filters, pigments and salts for skin, sun and make-up applications.
An additional advantage of this specific grade of PCP is that it is such a potent emulsifier that it provides the option to create low-viscosity but stable emulsions with a low emulsifier content, as these formulations show an improved sensory profile. And since a pleasant skin feel is a prerequisite for consumer compliance, it is proposed as a way to encourage consumers to use sufficient sunscreen to achieve better protection against harmful UV rays.
Figure 1. PEG-100 stearate structure
PEG-100 stearate produces many small, smooth areas of LCL structures with uneven edges.
Figure 2. PCP structure
PCP produces large, smooth LCL areas with smooth edges and sharp corners.
Figure 3. Micro-domains in PCP gel network (1,000 nm)
Small and round microdomains are visible on top of the lamellar structures, which seemed specific to PCP.
Figure 4. Micro-domains in PCP gel network (100 nm)
Small and round microdomains are visible on top of the lamellar structures, which seemed specific to PCP.
Figure 5. PEG-100 stearate TEM
With PEG-100 stearate, only LC lamellar structures are present.
Figure 6. Glyceryl stearate citrate TEM
With glyceryl stearate citrate, only LC lamellar structures are present.
Figure 7. PCP TEM
With PCP, LC lamellar structures plus LC vesicles are present.
Figure 8. Mixture of PEG-100 stearate and PCP (2,000 nm)
The mixture of PEG-100 stearate and PCP reveals large numbers of vesicles and micro-domains (2,000 nm)
Figure 9. Mixture of PEG-100 stearate and PCP (200 nm)
The mixture of PEG-100 stearate and PCP reveals large numbers of vesicles and micro-domains (200 nm).
Figure 10. Schematic of micro-domains
Schematic of the micro-domains found in emulsions based on the specific grade of PCP as the emulsifier
Figure 11. Lipid stabilization potential
Lipid stabilization potential of different emulsifiers after two months of storage
Figure 12. Stabilization of titanium dioxide
Stabilization of titanium dioxide comparing different emulsifiers after two months of storage
Figure 13. Stabilization of PBSA
Stabilization of PBSA comparing different emulsifiers at a use level of 1%, after two months of storage
Figure 14. Thermal stability after three months of storage
Thermal stability of sunscreen emulsions with and without the specific grade of PCP
Figure 15. Thermal stability after four weeks
Thermal stability of sunscreen emulsions with and without the specific grade of PCP after four weeks of storage
Formula 1. Sunscreen Emulsion 1
As a next step, PEG-100 stearate as a primary emulsifier and PCP as a secondary emulsifier were combined at a use level of 0.5% in an SPF 30 sunscreen emulsion.
Formula 2. Sunscreen Emulsion 2
A sunscreen emulsion with high oil loads and a targeted SPF 30 was formulated based on cetearyl glucoside.